Avogadro's Number Meaning
Mole and Avogadro’s Number
The chemical changes observed in any reaction involve the rearrangement of billions of atoms. It is impractical to try to count or visualize all these atoms, but scientists need some way to refer to the entire quantity. They also need a way to compare these numbers and relate them to the weights of the substances, which they can measure and observe. The solution is the concept of the mole, which is very important in quantitative chemistry.
Avogadro's number is named after an Italian Scientist, Amedeo Avogadro in the 19 th Century. As per his theory, at certain pressure and temperature conditions, the volume of gas is proportional to the number of molecules in the gas irrespective of the nature of gas. Because R is a constant, we can use the qualities of any gas — its temperature, pressure, volume, and number of moles — to determine the value of R. Avogadro’s law states that equal volumes of all gases, at the same temperature and pressure, have the same number of molecules.
Avogadro’s Number
Amadeo Avogadro first proposed that the volume of a gas at a given pressure and temperature is proportional to the number of atoms or molecules, regardless of the type of gas. Although he did not determine the exact proportion, he is credited for the idea.
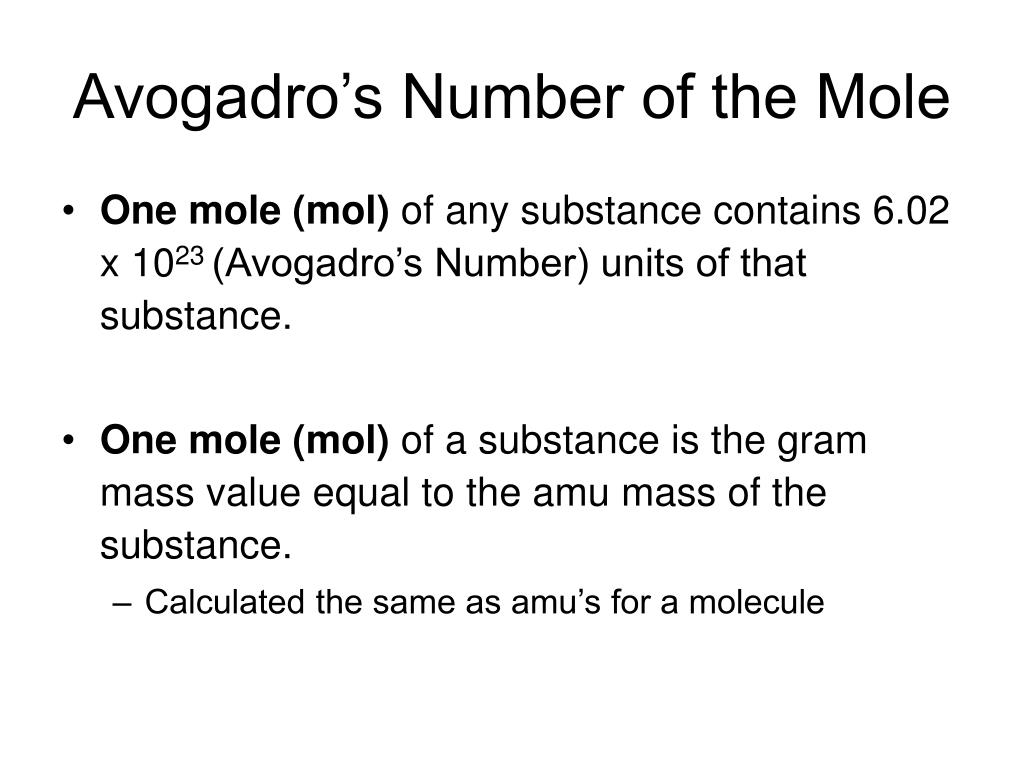
A mole is a quantity that contains 6.02 *1023 atoms, molecules and ions.
Avogadro’s number is the number of particles in a mole 6.02 *1023
The mole (or mol) represents a certain number of objects. The amount of a substance that contains the same number of entities as there are atoms in 12 g of carbon-12. One mole of H2O molecules contains 6.022 x 1023 molecules. • 1 mole contains 6.022 x 1023 entities (Avogadro’s number). One mole of NaCl contains 6.022 x 1023 NaCl formula units.
Exactly 12 g of carbon-12 contains 6.022 x 10 23 atoms.
*** There is a new definition of mole available at C & EN article
The meaning and usefulness of the mole: number of moles of elements in a compound can also be determined from the chemical formula. For example,
the chemical formula of water is H2O. Therefor in 1 mole of H2O contains 2 mols of H and 1 mol of O from the subscript of the chemical formula.
Mol and number of particles can be easily calculated by using the conversion factor:
1mol
6.022 x 1023 or vice versa
Examples:
How many atoms are present in 3.0 mols of Ag?
Ans: Since it is mols to atoms, Avogadro’s number is the conversion factor.
3.0 mols of Ag * 6.022 x 1023 atoms of Ag = 18.067 * 1023 atoms or 1.8 *1024atoms Ag
1 mol Ag
Avogadro's Number Meaning
- How many molecules are present in 2.93 mols of H2O?
Since it is mols to molecules, Avogadro’s number is the conversion factor.
Ans: 2.93 mols of H2O * 6.022 x 1023 molecules of H2O = 18.067 * 1023 atoms or 1.8 *1024
1 mol H2O
- How many mols of Hydrogen are in 1.56 mols of CH4?
Since it is mols from a compound to mols of an element, we will use chemical formula.
1.56 mols of CH4 * 4 mols H = 6.24 mols of H
- mol CH4
4) How many hydrogen atoms are there in 2.50 mol of glucose (C6H12O6)? - We will use both chemical formula and avogadro’s number to solve this problem.
2.50 mol C6H12O6 *12 mols H * 6.022 x 1023 atoms of H =180.66 x 1023 or 1.81 x 1023 atoms
1 mol C6H12O6 1 mol H
Watch the following video:
Questions:
- How many atoms are present in 5.5 mol of Fe?
- How many mols of each element present in 2 .00 mols of the following compound?
C9H8O4 (aspirin)
| 3. How many oxygen atoms are present in 5.20 mol of Al2(SO4)3? |
- How many molecules are present in 7.29 mols of the compound below?
5. 11* 1024 molecules are present in a glass of water. How many mols of H2O are present?


Ans: 1. 3.31*1024 atoms
2.18 mols of C, 16 mols H, 8.0 mols O

3. 3. 77 *1025 atoms of oxygen
4. 4.39*1024 molecules
5. 5.17 mols
SEE ALLAdd a noteAvogadro's Number Meaning Chart
Contrary to the beliefs of generations of chemistry students, Avogadro’s number—the number of particles in a unit known as a mole—was not discovered by Amadeo Avogadro (1776-1856). Avogadro was a lawyer who became interested in mathematics and physics, and in 1820 he became the first professor of physics in Italy. Avogadro is most famous for his hypothesis that equal volumes of different gases at the same temperature and pressure contain the same number of particles.
The first person to estimate the actual number of particles in a given amount of a substance was Josef Loschmidt, an Austrian high school teacher who later became a professor at the University of Vienna. In 1865 Loschmidt used kinetic molecular theory to estimate the number of particles in one cubic centimeter of gas at standard conditions. This quantity is now known as the Loschmidt constant, and the accepted value of this constant is 2.6867773 x 1025 m-3.
The Avogadro Number
The term “Avogadro’s number” was first used by French physicist Jean Baptiste Perrin. In 1909 Perrin reported an estimate of Avogadro’s number based on his work on Brownian motion—the random movement of microscopic particles suspended in a liquid or gas. In the years since then, a variety of techniques have been used to estimate the magnitude of this fundamental constant.
What Is Avogadro's Number
Accurate determinations of Avogadro’s number require the measurement of a single quantity on both the atomic and macroscopic scales using the same unit of measurement. This became possible for the first time when American physicist Robert Millikan measured the charge on an electron. The charge on a mole of electrons had been known for some time and is the constant called the Faraday. The best estimate of the value of a Faraday, according to the National Institute of Standards and Technology (NIST), is 96,485.3383 coulombs per mole of electrons. The best estimate of the charge on an electron based on modern experiments is 1.60217653 x 10-19 coulombs per electron. If you divide the charge on a mole of electrons by the charge on a single electron you obtain a value of Avogadro’s number of 6.02214154 x 1023 particles per mole.
Avogadro's Number Meaning Definition
Another approach to determining Avogadro’s number starts with careful measurements of the density of an ultrapure sample of a material on the macroscopic scale. The density of this material on the atomic scale is then measured by using x-ray diffraction techniques to determine the number of atoms per unit cell in the crystal and the distance between the equivalent points that define the unit cell (see Physical Review Letters, 1974, 33, 464).
